By Fabian Fernandez
Crop harvest is progressing quickly in Minnesota due to the dry conditions. As soon as the crops are out, it is time to start field activities to prepare fields for next year. One of these activities might include nitrogen fertilization.
What to apply?
In my recent blog post on nitrogen best management practices, I discussed the fact that, for fall applications, the only source that is reliable enough is anhydrous ammonia. This is because all other commercial nitrogen fertilizers have a high potential for loss when applied so far in advance of crop uptake. The reason anhydrous ammonia works for fall applications is that, when ammonia (NH3) is injected, it disperses in the soil, reacting with soil moisture (H2O) within a retention zone, which, depending on soil moisture, can be around three to six inches in diameter. As NH3 reacts with H2O, it forms NH4 and OH-. The OH- creates a temporary increase in soil pH that inhibits the activity of nitrifying bacteria within the ammonia retention zone. I would also strongly encourage the use of a nitrification inhibitor with a fall anhydrous ammonia application to delay, as much as possible, the process of nitrification. This is important because once ammonium is transformed to nitrate, it can be lost from the field.
Where to apply?
While our research conducted across many years and locations around Minnesota fairly consistently shows that fall nitrogen applications have greater risk of nitrogen loss (and reduced yields) compared with pre-plant or sidedress applications, fall nitrogen applications have some logistical and cost savings advantages that make it a preferred option for some farmers. If you are planning to do a fall application, nitrogen should be applied only in soils and situations with the lowest potential for nitrogen loss. Aside from soils with high leaching potential, where fall nitrogen applications are prohibited, I would also stay away from fields or portions of the field where water tends to accumulate or the soil tends to stay wet for prolonged periods of time in the spring. In these situations, the risk of nitrogen loss by denitrification can be very high.
When to apply?
While nitrification doesn't stop until the soil freezes at 32°F, once the soil reaches 50°F and keeps getting colder, the process slows down substantially. This has important practical implications because it provides a long enough window of opportunity to apply nitrogen before the soils freeze. Soil temperature should be measured where anhydrous ammonia is injected, typically at a 6-inch depth. A common misconception is that applying a nitrification inhibitor allows you to do the application in soils warmer than 50°F. That is simply not true because warmer temperatures make the inhibitor less effective by accelerating its breakdown.
Don't go by calendar date but rather use actual soil temperatures to determine when to apply nitrogen. You can obtain real-time soil temperature from various parts of Minnesota with the MDA's Six-Inch Soil Temperature Network map. That said, these temperatures should be used as a reference. The best approach is to measure the temperature of your fields before nitrogen application because many soil factors (such as soil color, amount of crop residue and drainage) influence soil temperature.
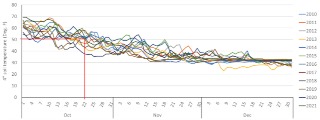
Unless rain in the forecast is going to keep you out of the field for a long while, it is often wise to wait a little longer after your soil reaches 50°F before applying anhydrous because, chances are that temperatures will warm back up again. As a point of reference (see figure), I looked at soil temperatures taken on bare soil at 4 inches below the surface at the Southern Research and Outreach Center in Waseca for the last 12 years (2010-2021). For that site, on average, October 22nd is when mean soil temperature gets to 50°F, but the range of temperatures on that day over the last 12 years has been between 37.5 and 57.8°F. That's why I said don't make plans based on a calendar date. Looking at the first day of each year when the soil temperature reached 50°F, I noticed that soon after, on average we get around 8 days of average soil temperatures above 50°F. Over the last 12 years, the earliest date when we reached 50°F was October 3 and the latest was October 26. I also noticed that anytime the soil temperature got to 50°F before Oct 15, the chance to have warm temperatures soon after for 12 to 21 days increased substantially. On the other hand, when the soil got to 50°F after October 20, the chance to have temperatures above 50°F at a later date was reduced to less than 3 to 7 days.
How to apply?
Anhydrous ammonia is liquid under pressure but it becomes a gas when injected in the soil. If the soil is too dry or too wet, it can result in nitrogen volatilization losses. As mentioned earlier, when anhydrous ammonia is injected, it travels through the soil pores, reacting with soil water. The drier the soil, the greater the distance ammonia will travel to react with water. If the ammonia retention zone is larger than the depth of application, you can end up with ammonia escaping into the air because it ran out of room to react with soil water. If the soil is too wet, the knife track might not seal properly and ammonia will escape through the opening left by the knife. For either condition, the easiest way to know if you are losing nitrogen by volatilization is to apply a strip and, after a few minutes, check to see if you smell ammonia. In dry soils, sometimes, but not always, increasing the depth of application can help resolve this issue. Finally, you should apply anhydrous ammonia with extreme caution. Anhydrous ammonia will react quickly with the moisture in your skin, eyes, or mucous membranes, causing dehydration and burns. Don't be careless when working with anhydrous ammonia.
How much to apply?
To make the most profitable decision, the corn nitrogen rate calculator provides the economically optimal nitrogen rate at various corn and nitrogen prices. The data used for this calculator comes from trials around Minnesota with nitrogen applications done in the spring to minimize nitrogen loss. As I mentioned before, spring applications are normally more efficient than fall applications. Applying more nitrogen with a fall application to overcome the lower efficiency is not a good practice because nitrogen is too expensive and nitrogen loss contributes to environmental issues. It is good to remember that you don’t have to apply all of your nitrogen in the fall. If you are risk-averse but a fall application makes sense for your operation, consider applying a small portion of the total nitrogen requirement in the fall and applying the rest in the spring. The reality is that the crop does not need very much nitrogen to get going in the spring. Applying the remainder closer to when the crop needs it can increase your overall nitrogen use efficiency.
Source : umn.edu