Many farmers are wondering the same thing this time of year: How much of my fall-applied nitrogen is still available in the soil? To answer that question, we need to consider the nitrogen cycle. Here’s a quick refresher.
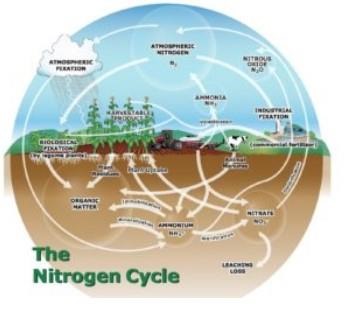
Three dominant forms of nitrogen exist in the soil: ammonium (NH4+), nitrate (NO3-), and nitrogen (N). Plants mostly take up ammonium and nitrate nitrogen for their growth, while nitrogen contained within soil organic matter is a slow-release source of ammonium in the soil.
Anyone who has had a soil test done is familiar with the CEC (cation exchange capacity) of their soil. The CEC measures how well the soil can hold positively charged nutrients, which is important to know because soil particles – sands, silts, and especially clays – have many negative charges on their surfaces, and very few positive charges.
Think of the soil and the different forms of nitrogen as the charged ends of a magnet. The ammonium form of nitrogen is positively charged, so the negatively charged soil particles will attract ammonium and easily retain it in the soil. The nitrate form of nitrogen is negatively charged, so it is not attracted to the negatively charged soil particles. Because nitrate also dissolves easily in water, it tends to move wherever water moves in the soil. This is great for a crop that is actively pulling a lot of water out of the soil, but also means that nitrate can be easily lost if water in the soil is moving down to a tile drain or groundwater.
Nitrogen in the soil tends to change forms in fairly predictable ways. The nitrogen forms commonly found in fertilizers, like anhydrous ammonia and urea, convert to ammonium in soils within a few days of application. In the process of nitrification, microorganisms in the soil will then convert ammonium to nitrate over time. These microorganisms are most active in moist, warm soils. Under those conditions, most fertilizer nitrogen usually converts to nitrate within a few weeks. In cases of flooded soils without oxygen (like wetlands), or frozen soils, fertilizer nitrogen will not convert to nitrate. Since Minnesota’s agricultural soils only rarely become depleted of oxygen, the net result of nitrification is an accumulation of nitrate in our soils during the growing season.
The nitrate that accumulates in agricultural soils has a seasonal pattern to it. Research plots have shown that, where nitrogen rates have been optimized for corn production, nitrate accumulates in the soil in the spring, after fertilizer application, as soil temperatures warm up. Typically, where nitrogen fertilizer has been applied preplant, nitrate levels spike between corn emergence and the V6 growth stage, and then decline steadily until the crop has matured. The soil is still producing lots of nitrate from organic matter later in the growing season, but by the V8 growth stage, corn is rapidly removing nitrate from the soil. In optimally-fertilized corn, it is not unusual or alarming for a soil that had above 25 ppm nitrate at V6 to have less than 5 ppm by R1.
Click here to see more...