The presence of a mineral in forage, water, feed, or supplement does not constitute utilization of the mineral in cattle bodily processes. Understanding the bioavailability of mineral sources in cattle can help producers maximize productivity in their operations.
Mineral nutrition in beef cattle is a complex subject. Minerals must be supplied in a balanced manner to prevent common problems such as oversupplementation (toxicity), undersupplementation (deficiency), or imbalanced ratios with other minerals. Striking the right balance is easier when a producer understands the bioavailability of a mineral source to be ingested, absorbed, retained, and utilized by the body.
Fortunately, novel laboratory testing procedures have offered new insights into mineral utilization and their effects on tissues and metabolic processes of the body. This has led to advances in feed supplementation that include more efficient means to supplement minerals to cattle and protection of high-producing cattle from emerging diseases such as chronic oxidative stress seen in feed yards.
Bioavailability Basics
Early work on the bioavailability of minerals focused on macro minerals. As more information about the importance of trace or microminerals has become available, researchers have shifted focus to them. Mineral sources are divided into two major categories: inorganic and organic compounds. Not every molecule within a source group has the same bioavailability. For instance, copper oxide and magnesium oxide are both oxides but have very different bioavailabilities.
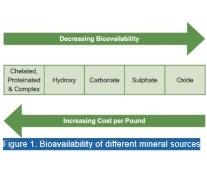
Figure 1. Bioavailability of different mineral sources
Inorganic mineral sources are mainly metallic salts that are loosely bonded and separate readily in water. Examples of this group are oxides, sulphates, carbonates, and chlorides. The notable exception is the hydroxide sources. While still considered to be an inorganic source, hydroxide molecules are more tightly bonded together and require more acidic solvents for the separation of the elements.
Organic compounds have elemental metals attached to proteins, peptides, or amino acids. They are often small enough to be absorbed whole and used in body functions or are broken down before absorption.
While there are notable exceptions to the rule, the availability of different mineral sources would be on a continuum resembling the one shown in figure 1. The mineral sources shown here are those most likely to be found on retail mineral tags.
Inorganic Minerals
Oxides
Oxide mineral sources have been used since the mid-19th century when potassium was identified in the residue of tissue combustion research and was nicknamed “potash,” a name that remains popular today. These mineral sources are bonded to form metal salts, such as potassium oxide and copper oxide. These compounds have a strong bonding affinity and often pass through the animal unused, indicating a low apparent bioavailability. Apparent absorption of most oxides is less than 30 percent, with some being close to zero.
Oxides are usually a cheaper source of minerals due to abundant supplies and lower bioavailability potential. The most notable exception to this is magnesium oxide (MgO). MgO is commonly used as a source of magnesium to ruminants because it is inexpensive and can be 30 to 60 percent bioavailable. MgO, like other oxides, can have a metallic taste and not be readily consumed by cattle.
Variability in MgO bioavailability has been identified between products from different manufacturers as well as between batches of the same product from the same manufacturer. This variability may be caused by particle size and rumen pH at the time of consumption.
Sulfates
Sulfates, like oxides, have been used quite extensively to supplement needed minerals to cattle. Their popularity grew after new technologies were discovered around the time of World War I. These newer technologies created a more bioavailable and cheaper source of minerals. Used for the last 70 years, sulfate mineral sources have become the standard when testing the bioavailability of sources.
Copper sulfate is one of the most recognized forms utilized in mineral programs today. The metal in this group is loosely bonded to the sulfate group. The metal also separates easily in solution and can create some challenges in the rumen with trace mineral absorption.
Some elements attached to the sulfate group can have negative impacts on rumen bacteria when released due to their antimicrobial effects. In addition, these elements can form insoluble compounds with other constituents of the rumen before reaching the site of absorption in the small intestine, rendering them useless to the animal.
Carbonates/Chlorides
Carbonates and chlorides are compounds commonly used for mineral supplementation. Table salt (NaCl) is probably the most recognized ingredient in chloride form. Salt separates almost completely in the rumen and is completely available to the animal. Macrominerals such as calcium (Ca), magnesium (Mg), and sodium (Na) are often supplemented in the carbonate/chloride form. This group is often higher in bioavailability than the oxides and similar to the sulfate group.
Hydroxy
Hydroxy minerals are a newer form of mineral that comes as a by-product of the electrical industry. Like the previous three groups of mineral sources, this group is still considered an inorganic molecule, even though it shares some commonality with organic compounds.
This group is different in that it has a tighter bond to the metal holding it together through the neutral pH of the rumen. This technology has been used mainly in microminerals, such as copper (Cu), manganese (Mn), and zinc (Zn), to provide a more targeted supplementation profile. Because of the tighter bonding, the compounds can stay together until they enter the more acidic area of the abomasum (true stomach of cattle) right before the small intestine.
The site of absorption for most microminerals is the middle third to the end of the small intestine. The metals attached to the hydroxy group are not as available in the rumen to create inert products that are unusable by the cattle; it is therefore suggested that more of the minerals are available closer to the site of absorption, increasing the apparent absorption.
By bypassing the rumen, trace elements such as zinc and copper do not have the antimicrobial actions seen with sulfates and oxides. In addition, hydroxy compounds are more palatable than other mineral forms.
There is an increase in cost per pound when using hydroxy minerals as a substitute for one of the previous three groups. This increase may be nullified if more mineral is available to the animal, allowing a lower feeding rate.
Organic Minerals
Organic minerals are referred to as chelates, proteinates, or complex on mineral tags. These compounds are challenging to make and typically are a more expensive mineral source.
The American Association of Feed Control Officials (AAFCO) gave the first definition of a chelated mineral in 1970, allowing it to be used in animal feeds. However, knowledge of the chelation process dates back to the late 19th century. This definition was later revised by AAFCO to a more stringent definition based mainly on molecular weight. This was because many manufacturers were using chelated metal compounds that had not been proven to be an effective supplement source for cattle.
Like hydroxides, organic mineral sources are tightly bonded and do not separate easily near neutral pH, like in the rumen. Organic minerals have the mineral attached to larger molecules, such as peptides, amino acids, or proteins.
There is evidence that organic mineral forms can be absorbed through different pathways than those of inorganic minerals. When inorganic mineral sources are used, organic minerals do not compete for absorption sites. This may allow a higher amount of minerals to be absorbed with lower intakes.
Organic minerals differ from inorganic minerals in several ways. These compounds are typically absorbed whole, whereas inorganic compounds require separation before the metal can be absorbed. This characteristic reduces the chance of interference of other compounds that would steal the metal to make an unusable compound. Once absorbed, the organic molecule can be used in the body in several ways.
Summary
Mineral supplements can be formulated using multiple sources of minerals. Each mineral source has advantages and disadvantages. The decision to use a specific mineral product over another should be made after careful consideration:
- A mineral source must be palatable to cattle so that they will readily consume the required amount per day. A mineral supplement that cattle will not consume at the recommended levels is just as detrimental to the operation as a mineral that is overconsumed on a cost-per-head basis.
- A balanced mineral program consumed at the proper level should alleviate any deficiencies or potential toxicities and meet the minimum requirements of the animal being supplemented.
- The level of production expected and the cost-to- benefit ratio of each operation must be determined to ensure the animal requirements are met at an acceptable cost when deciding which mineral sources to use.
Due to the low feeding rate of most mineral products, the relative cost per head per day is very similar on a wide range of mineral products with different technologies and mineral sources included. For instance, the 4-ounce mineral intake of a product that is priced at $20 a bag costs $0.10 per cow per day. The 4-ounce mineral intake of a product that is priced at $31 a bag costs $0.155 per cow per day. The difference between the two mineral products is $18.25 per cow per year. The real question becomes what level of production or reproduction is required to offset the added expense. That must be answered on an individual operation basis. Consult with a nutritionist to help you understand which mineral supplementation program fits your operation best.
Source : aces.edu