By Dan Kaiser and Leanna Leverich
Potassium (K) is needed by all plants and potassium deficiencies can significantly reduce crop yield. While the chemistry of potassium in soils is relatively simple, K availability can be significantly impacted by environmental conditions. If soils are dry, K deficiencies can occur. Plant roots need water to efficiently draw K from the soil, which has been in short supply the last two years. But there are other things that can impact K availability as well.
Cation exchange capacity
Potassium is taken up by plants as the potassium anion (K+). Cation exchange capacity (CEC), or the soil’s ability to hold cations, including the K+ ion, can also impact K availability. The soil’s CEC is influenced by the abundance and type of clay present. Most of Minnesota’s soils are classified as “mixed” clay mineralogy, but most farmers’ fields are dominated by one clay type. Soils high in illite clays tend to have a low shrink-swell capacity and a lower CEC compared to soils high in smectite clays, which tend to shrink and swell. What is not known is how these clay types influence K availability in the soil.
Sandy soil
Sandy soils represent a separate challenge as sand itself has no CEC and many of the glacial outwash soils in Minnesota have very little clay giving most sandy soils a very low CEC. In fact, while the K+ ion is not particularly mobile in high clay soils, potassium can leach in sandy soils. This makes it much harder to rely on “leftover” K not used by the previous crop to be available for the following crop.
Potassium guidelines for corn and soybean
Recent research has shown that it takes less K to maximize corn and soybean yield in some sandy soils. This is part of the reason why we are currently re-evaluating the university’s potassium guidelines for corn and soybean.
Over the past three years, we have begun to look at how crops grown on different soil types respond to potassium. Funding from the Minnesota Corn Research and Promotion Council has made it possible for us to look further into clay mineralogy across the state. There are three key areas we are looking at in this project.
- How does clay mineralogy vary across the state? More specifically, we are looking at how soil clays vary in their illite and smectite content, and whether this variation can be used to refine our potassium guidelines. In this process, we are also looking at CEC to determine what might be the best method for predicting crop K needs.
- Why do we not find a large response to potassium in sandy soils that test low to very low in K? We are trying to determine whether K is being released through weathering of soil minerals and how changes in soil pH impact CEC, which could potentially affect soil K retention.
- Should we update our potassium fertilizer rate recommendations and maintenance-based K guidelines? Once we know more about which soil properties impact K availability, we will re-evaluate current K fertilizer rate recommendations and suggested soil critical levels to be used for those interested in a maintenance-based K strategy.
Minnesota potassium mineralogy map
We currently need help to complete the first part of the study, which involves mapping soil K mineralogy in Minnesota. The map below shows where data has been collected and where soil samples have been taken but not yet sent in for analysis.
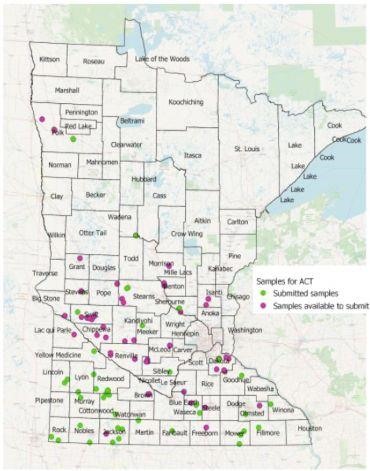
We still need soil samples from these locations in Minnesota:
- Southeast: Wabasha, Dodge, Houston, Rice, Le Sueur (north), Blue Earth, Nicollet
- Southwest: Watonwan, Martin, Pipestone, Lyon (north), Redwood (northeast), Yellow Medicine (west)
- West-central: Kandiyohi (north), Pope, Stearns (east)
- Northwest: Traverse, Douglas, Todd, Otter Tail, Wadena, Becker, Wilkin, Clay, Norman, Mahnomen, Clearwater, Red Lake, Pennington, Marshall, Roseau, Kittson
- East/Central: Wright, Anoka, Chisago, Mille Lacs, Kanabec, Washington, Eastern Hennepin
The goal of this study is to get at least one to two soil samples from each of the major agricultural areas of the state. We are still in need of soil samples from the majority of the Red River Valley as well as some locations in west-central Minnesota and the far southeastern and southwestern corners of the state.
Source : umn.edu