By Bob Hartzler
Several reports of new resistant traits in the weedy Amaranths were announced recently. First, Illinois reported
two populations of waterhemp resistant to metolachlor and other Group 15 herbicides. This was followed by reports of metolachlor and then 2,4-D resistance in Palmer amaranth by
Arkansas and
Kansas, respectively. The Kansas Palmer amaranth population is also resistant to dicamba. Although only Illinois has confirmed the resistance mechanism in these populations is metabolism-based, there is a good likelihood that each of these cases is due to the weeds ability to rapidly degrade the herbicide. In this article I will review herbicide metabolism by plants, and discuss why metabolism-based resistance poses a bigger threat than target site-based resistance. Less is known about many aspects of metabolism-based resistance than with target site-based resistance, and in-depth papers detailing the newest resistance cases have yet to be published. Thus, some of the information below is speculation on my part.
Basics of metabolism-based resistance
Herbicide metabolism is typically described as a three-phase process, although some herbicides don’t require the first step (Figure 1). Phase I involves a slight modification of the herbicide molecule which predisposes it to further modification. Phase II involves combining the modified herbicide with another compound (sugar, glutathione, etc.) that facilitates the final step. Phase III involves transport enzymes that move the herbicide into the cell vacuole (often described as a cell’s garbage can) or outside of the cell into the intracellular space. Movement of the herbicide to these areas isolates the herbicide from the target site. Some herbicides are further degraded in the vacuole.
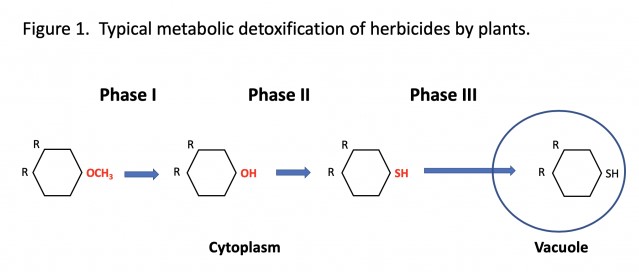
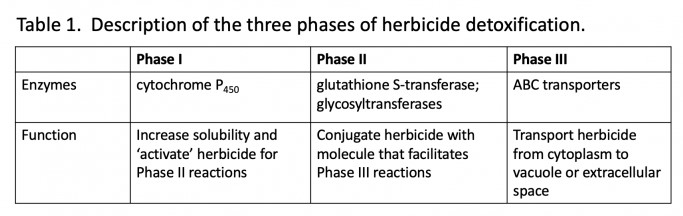
Both plants and animals use the same enzyme families for these processes (Table 1), but plants typically have more versions of these enzymes than animals. For example, rice has more than 360 genes for cytochrome P450 enzymes (Phase I reactions), whereas humans have less than 60. The large number of genes allows an organism to process a wide variety of molecules. Plants probably have more genes for these enzymes than animals since they have less ability to control their exposure to toxins. Animals can modify their diet to reduce the intake of toxins, or physically leave areas with toxins – plants don’t have these options.
The major difference between detoxification of chemicals in animals and plants occurs in Phase III. As mentioned, plants store the modified herbicide in areas where it doesn’t pose a threat. In animals, Phase II reactions increase their ability to filter toxins from the blood via the kidneys. After removal of the herbicide from the blood, the compound can be excreted from the body via urine. If you’ve ever wondered why waste of animals fed forage from areas treated with picloram (Tordon, Grazon, etc.) or other persistent Group 4 herbicides can result in injury to sensitive crops, this is why.
Many herbicide formulations include safeners that increase the crop’s tolerance to the herbicide by enhancing metabolism by the crop. Several Group 15 products (Dual II Magnum, Harness, Surpass, et al.) include safeners that enhance the activity of glutathione S-transferase (Phase II). Accent Q, Balance Flexx, Corvus, Status and other products include safeners that increase the activity of cytochrome P450 (Phase I).
Why should we be concerned about metabolism-based resistance?
Nearly all of Iowa’s herbicide resistance problems prior to 2005 were target site-based (Table 2). Most involve a simple alteration to the site of action that reduces binding of the herbicide to the target site. Glyphosate resistance in waterhemp is due to overexpression of the target site, which dilutes the effect of glyphosate. In the past 15 years, metabolism-based resistance in waterhemp has been reported for the following herbicide groups: 4, 5, 15, and 27. As mentioned earlier, several new cases of resistance that are likely metabolism-based were recently reported.
The concern with metabolism-based resistance is that an alteration in herbicide metabolism providing resistance to one herbicide group may provide cross-resistance to several classes of herbicides. This is well documented in Australia; widespread metabolism-based resistance has led to the development of
harvest weed seed control and other alternative weed management strategies.
The recently reported metolachlor resistant waterhemp (IL) and 2,4-D resistant Palmer amaranth (KS) occurred in populations that were already resistant to Group 27 herbicides. Illinois researchers previously reported a Group 27 resistant waterhemp that was resistant to 2,4-D. Group 27 resistance in waterhemp is due to increased activity of cytochrome P450 enzymes. Metolachlor and 2,4-D resistance in other weed species has been linked with increased activity of cytochrome P450. It is possible, if not likely, that the genes providing resistance to the Group 27 herbicides are responsible for the new resistances in the recently identified populations. It is important to note that not all Group 27 resistant populations have resistance to these other herbicide groups. Dr. Owen’s group screened several populations of Group 27 resistant waterhemp from Iowa and did not find any with 2,4-D resistance.
We have moved into an era where metabolism-based resistance will increase in importance. The potential for metabolism-based resistance to provide cross-resistance to herbicides that have not been heavily relied on in the past is a real threat to our current production system. It is critical to develop herbicide programs that: 1) rely on multiple effective herbicide groups, and 2)
provide full-season control. However, herbicides alone cannot win this battle. Production systems must be evaluated to determine what alternative strategies can be used to supplement herbicides. While strategies such as increased crop competitiveness via narrow-row spacing or planting cover crops do not provide the ‘big impact’ of herbicides, their contribution to suppressing weeds can make the difference between long-term success or failure in weed management.